
If I may oversimplify, clay melts if you get it hot enough. Primary kaolins are typically pure enough that they melt only at very high temperatures, but many darker clays have enough iron and other easily-melted materials in them that “hot enough” is well within the pottery range.
Many of these clays make eminently satisfactory glazes either by themselves, or mixed with the right Other Things. What those Other Things are, of course, depends upon the temperature at which you are cooking your pots. (In my case, because I work at 1300 celsius or so, I can add: nothing; feldspar; silica; iron oxide and/or other colorants; other clays; wood ash, either natural or synthetic; other glazes; and so on.)
Perhaps the most well-known clay that was used primarily in glazes during the past century or so was something called Albany Slip, which was dug at or near Albany, New York. If you’ve seen old brown insulators like these

either on guy-wires or telephone poles, chances are that they were covered with an Albany Slip glaze. Albany Slip was used by potters, too, and many of them loved it. You can still find lots of old glaze recipes that call for it.
...But it’s gone.
Because Albany Slip is no longer being dug commercially, people have made various equivalents by starting with other clays and making judicious amendments to them. The object is to get something that you can substitute straight across, so you don’t have to develop your glaze recipe all over again from scratch with different materials. In order for this to work, you either have to have rock-solid (if you will excuse the expression) analyses of Albany Slip and your starting material, or exquisite examples of Albany Slip glazes, the recipes for them, and detailed information about how they were fired... and in either case, lots of patience is recommended.
One obvious issue here is the fact that Albany Slip was not just one unchanging material. I have 5 analyses, no two of which are precisely alike. This has the advantage of giving you a certain amount of latitude in the composition of your substitute, so the “rock solid” requirement I mention above is something of an exaggeration. (I couldn’t resist.)
Some people think it’s no good to attempt to replicate materials that have gone missing. It’s better, they argue, to use what’s available, and do what you can with it. I have no particular axe to grind, myself — if someone wants to use Seattle Slip, Alberta Slip, or some other approximation of Albany Slip, more power to them. There are, of course, some materials for which no commercial substitute exists, and it can be ...interesting to attempt to copy them. That, however, is a whole ’nother story.
Here, anyway, I must in good conscience admit that I have a very special reason for not worrying about this issue: I’ve got something that gives me glazes I love so much that I am not particularly concerned with Albany Slip or its imitations and substitutes.
You see, I have a friend who lives on top of millions of tons of clay. (I’ve mentioned this before, I think.) This clay is relatively ordinary stuff, as far as one can tell by looking at it:

On the left is a wee lump of the stuff, as dug. I broke it off a much larger lump. On the right is what it looks like after I run it through a 10-mesh sieve and then a 40-mesh sieve. To the naked eye, it closely resembles powdered chocolate milk drink mix. (I actually have some Albany Slip, and it looks very similar.)
In what you are going to see below (as I get this page
built, which may take me a little while), I use, for the
most part, three ingredients: Slip Clay, Wood Ash
[unwashed], and Red Iron Oxide. I have, on some
occasions, also added Silica, but that does not seem to
help; the clay itself is already fairly high in Silica
and low in Alumina. There will probably be a few tests
in which I use just clay and ash, or just clay and red
iron oxide, and if I have time I may put up one or two
in which I use feldspar or petalite in place of the ash,
but under ordinary conditions it’s those three
things.
Further info, in case you give a hoot:
The numbering system I’m using divides the total contents into 20 parts, so a change of 1 is a change of 5%. Thus, “XX00” (I’m using X for 10) is 50% clay and 50% ash. I haven’t actually made that mix, but I did make 17.300. (You’ll see a photo of it below.)
If I get a chance to make tests with natural ash,
I’ll probably precede the numbers with
“N”. Otherwise, I’m using a synthetic
wood ash that I bought at Seattle Pottery Supply. It is
apparently intended to be similar to a generalized
unwashed wood ash. [Some people like to wash their wood
ash, because they are primarily interested in the effect
you get with a material that is low in soluble fluxes
and high in calcia. That is not where I’m headed,
and I have no interest in removing the fluxes from this
material, so I never wash actual ash. Moreover, it would
not make any sense to wash the synthetic stuff that I am
using for these tests.]
Here are some tests I’ve made:





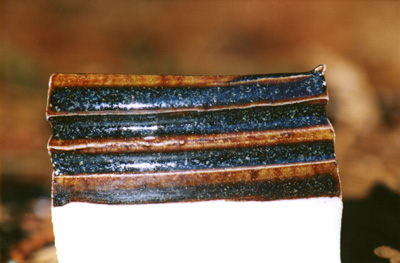
One other thing: I started out using clay that had been sifted only through a 10-mesh geological sieve. When I mix the glazes that way and then run them through a 60- or 80-mesh sieve I lose a lot of material, most of which appears to be sand. (No big surprise there.) Unfortunately, the precise amount is uncertain, which makes it extremely difficult to reproduce the recipes with any precision. For the recent synthetic ash tests, however, I’ve been running the dry clay through a 40-mesh sieve. Unfortunately, I failed to do that on the 17.300 test, so it is slightly suspect. When I get to the east coast I will probably acquire a large 60-mesh sieve, and I’ll run a lot of the dry clay through that.
[[Note, added in proof, much later: I have mostly been running the clay through an 80-mesh sieve, but as a liquid slip — it is nearly impossible to run dry clay through an 80-mesh sieve at any reasonable pace. I have also used a 120-mesh sieve for several batches, and a 150-mesh sieve for one batch, but I have some question as to whether I can get the red color I want with the finer material. Please see my recent TJIIRRS column about this for more information. (If 020 is the wrong number, just jump back to the Index and find the correct one — I shift things around on occasion, and don’t always catch the links from other pages.)]]
Fireplace ash, of course, contains lots of large particles
even after I put it through the 10-mesh geological sieve,
and I lose quite a bit of those, when I run the glaze
through a 60-mesh sieve after I mix it. My admittedly
limited experience is that if I just run the ash through
the coarse sieve, and do not run the glaze itself through
the fine sieve, I don’t like the way it looks after I
fire it. As you’ll see in a moment, however, it looks just
fine if I add clay and iron oxide and then don’t sift it.
Here (please excuse the pebble under the jar -- I should have hidden it) is a piece with the usual four-ingredient glaze (clay, fireplace ash, silica, and red iron oxide). It is in the collection of D. Potter, and shows the glaze just before I added more clay and some red iron oxide, a couple weeks ago. When I did that, I got this back as my first glaze test. Fortunately, I hadn’t had time to run the result through a sieve after I added the stuff, and I was very happily surprised by the little dots and splashes of color.
I’m probably going to run the glaze through a sieve, now that I’ve added a quantity of ash to it, because every time I dipped another piece into the glaze I had to stir it up. Had I been able to dip three or four in one session, things might have been different; but the stirring eventually broke up the clumps of iron oxide and of clay, so the splashes of color mostly went away. On the other hand, I’m likely to check future test glazes before I go running them through a sieve!
The fireplace-ash glaze has gone through various changes since I first mixed it. My first teapot is an example, as is the teacup on that same page. Both of those, in addition to the glaze itself, make use of an overglaze wash consisting of equal amounts of ceramic rutile and gerstley borate. On some glazes, such a wash is scut-awful, but on many tenmoku glazes and similar things, it is quite pleasant. (I’ve also had mild but happy results from a wash consisting of equal amounts of ceramic rutile and wollastonite, applied over a kaki glaze. I believe it was the old standard Ohata version, which you can find in various books.)
I should probably mention the fact that natural plant ash is insanely variable in composition. I typically burn one particular brand of fake fireplace "logs"; these are made of California cedar, I think, and give an ash that is relatively uniform. I’m not enough of a purist, however, to worry about bits of paper or modest quantities of other kinds of wood that may get into the mix.
I have been fortunate enough to get an EDAX analysis of a sample of this clay. I haven’t fully chewed the results yet, so it isn’t as cut-and-dried as saying “63.4% silica”, but I should eventually be able to derive that sort of detail from it.
(Note, added somewhat later: I have since gotten a
regular analysis of this clay. It turns out to be
almost 75% silica, actually a fairly high value, and
it is relatively low in alumina.)
To my Joss Research Institute report on Red Temmoku
Pseudo-mailto: jon [at] bazilians [put it here] org
Last modified: Mon Jan 29 20:07:45 PST 2001