(11 October, 2007)
The first coumarin in wide use was 7-Diethylamino-4-Methyl-Coumarin, often called Coumarin 1. (Exciton lists it as Coumarin 460.) It lases in the indigo and blue, with a tuning range of about 437 nm to 485 nm, depending on the pump source. Since Coumarin 1 was discovered, quite a few other Coumarin compounds have been found to be good laser dyes; the most common ones offer good performance across a significant portion of the visible spectrum, roughly 420-620 nm. Many (if not most) of these dyes are amino-substituted, but there are also hydroxy-substituted coumarins that can be lased. This page describes the best-known member of that group. (More specifically, the Umbelliferones, which have a hydroxyl group at position 7.)
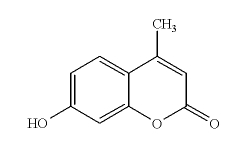
7-Hydroxy-4-Methyl-Coumarin, usually called 4-Methyl-Umbelliferone (I will refer to it here as 4-MU, for brevity) dissolves well in ethanol and isopropanol, but is not easy to lase in neutral or acid solutions. Two main characteristics distinguish this dye. The first is the fact that it has the widest tuning range of any ordinary laser dye: from 370 to 580 nm (!), in acid solution. (See the section on Tuning, below, and the references at the end of this page.) The second is that in basic solution it is very easy to lase. Even in basic solution, it has a relatively wide tuning range. Here is one version of the structure as it would exist in a neutral solution:

Below are some photos of 4-MU fluorescence. The same stock solution (in 99.8% isopropanol) was used for all of them, and the amounts of acid and base that I added were small, so the dye concentration is roughly the same throughout the series. In the three pictures on the left, the solutions are illuminated by a filtered LED flashlight with peak emission near 380 nm, which was very kindly given to me by Gordon Garb. In the three on the right, the solutions are illuminated by an ordinary 4-watt “BLB”-type fluorescent “blacklight” bulb, which is visible at the right edge of the bottom photo.
At the top, a drop or two of 85% phosphoric acid has been added to the stock solution, to acidify it. In the middle is the stock solution with nothing added to it, so it is essentially neutral. At the bottom is the solution with 1 drop of concentrated (about 29%) ammonia added, to make it basic.
Some things to note: first, the fact that there is a slight color difference between the top two photos; the the one on the right is a little bit more green than the one on the left. This is not easy to see here, but is quite apparent to the eye; the color is even more greenish when the solution is illuminated with 337 nm from a nitrogen laser. The significance of the color shift is that the fluorescence peak of 4-MU in acid solution is noticeably dependent on the excitation wavelength, a characteristic that is not particularly common. It suggests that, especially under broadband pumping (for example, by flashlamp), an acid solution may have a wider tuning range than either a neutral solution or a basic solution. I have not been able to lase 4-MU in acid solution yet, but I have only tried it with a very small TEA nitrogen laser; when I have the time, I will be trying it in a flashlamp-pumped laser.
(The acidic solution appears to be slightly brighter than the neutral solution in these photos, but that may be an artifact; the camera adjusts the exposure automatically. The white balance, however, was fixed, and did not change from picture to picture.)
Next, the last pair of photos: when even a small amount of strong base is added to 4-MU the fluorescence becomes much brighter, as you can tell (despite the camera’s attempt to compensate) by the light reflected from the paper towel that the cuvette is sitting on.
Finally: the absorption is more pronounced in basic solutions. The light from the LED flashlight goes all the way through the cuvette in the middle photo, but barely gets into the solution at bottom. This is crucially important for nitrogen laser pumping. A basic solution of 4-MU in isopropanol or ethanol is extremely easy to lase with either nitrogen-laser or flashlamp pumping.
Here is one more photo, showing the solution before I mixed it, just after I added the drop of ammonia. The effect of adding base is readily apparent:
(30 May, 2004)
Note: I culled the tuning photos below from three series, all of which I took this evening. The tuning range of 4-MU in my setup is wide enough that I can’t reach all of it without manually rotating the mirror mount that carries the diffraction grating — the adjustment range of the mount itself is not sufficient. Even so, I have not reached the published limits, though I should note that the widest tuning range for this dye, something like 117 nm (!), is achieved only in acidic solutions. (I have not found it at all easy to lase 4-MU with acid added, rather than base; that may have to wait until I have a more powerful pump laser.)
First, here’s a quick look at the dye laser:
This setup is extremely casual, but it fulfills one crucial requirement for tuning when a TEA nitrogen laser is used as a pump source: the round-trip transit time for light inside it is probably about 100-120 psec, so the light can bounce back and forth between the mirror and the grating perhaps half-a-dozen times during the pump pulse, which comes from a room-pressure nitrogen laser, and is less than a nanosecond long. This provides enough feedback to tune the dye laser, as the photos below demonstrate. (If the transit time of the dye laser is too long there won’t be enough feedback, and the output won’t be tuned well. This is less of an issue with ordinary nitrogen lasers, which operate at reduced pressure and therefore put out pulses that are several nsec long, than it is with TEA nitrogen lasers.)
I can assure you that these photos fail to do justice to the colors, which are rich and speckly. There is also the fact that a camera just doesn’t pick up violet the way your eye does, and displays don’t generate violet, so the short-wavelength end of the spectrum cannot look realistic. Purple is nice, but it isn’t the same.
I should note that this series does not actually reach the violet end of the tuning range — I can tune down to perhaps as low as 420 nm with this setup, but 4-MU should in principle be capable of operation all the way to 400 nm or even less with nitrogen-laser pumping.
I should also note that the ASE (Amplified Spontaneous Emission) from the dye, which appears as a violet band or cloudy triangle in some of these pictures, looks more like a partial rainbow to the eye when the tuning is at the violet end of the range, though of course it lacks the orange and red wavelengths. I’m not sure why only the short-wavelength edge of the rainbow shows up here. (You’ll notice, btw, that the ASE largely disappears near the peak of the tuning curve. This is expectable: the dye lases really well at those wavelengths, so most of the excitation goes to produce tuned output.)
One other rather peculiar thing I’ve noticed with
4-MU: when I first mix the solution (at least when
I’m using 91% isopropanol as the solvent;
don’t recall whether it’s the same with 95%
ethanol, though I would presume that it is), it has a
reasonably wide tuning range. After the solution sits
for perhaps half an hour, however, I find that the
tuning range has broadened significantly. I can only
guess that either the dye-ammonia complex takes a while
to form fully, or perhaps some sort of dye-ammonia-alcohol
complex forms in the solution (again, rather slowly).
...That, or something truly arcane is going on. I do
not have access to an NMR spectrometer, so I am not
easily able to determine which of these is the case.
(18 November, 2009)
Although it is not as easy to lase 4-MU in neutral or
acid solution as it is in basic solution, it is possible
if you have a nitrogen laser with good peak power. Here
is an [extremely informal] photographic tour of the
tuning curve of 4-MU with citric acid. I was not able to
reach the published tuning limits, but I suspect that
with some diligence and with phosphoric acid rather than
citric, I could get fairly close. (If I do so, I will
probably substitute a new set of photos.)
Some notes:
Physics of Dye Lasers
Near UV to Yellow Tunable Laser Emission from an Organic Dye
Characteristics of the 4-Methylumbelliferone Laser Dye
Another nitrogen-pumped dye laser page
To the top of the LASERs section
Email: a@b.com, where my first name (just jon, only three
letters) replaces the a, and “joss” replaces
the b.
Phone: +1 240 604 4495.
Last modified: Tue May 9 11:53:24 EDT 2017
2. Tuning 4-Methyl-Umbelliferone in Acid Solution











References:
C. V. Shank
Reviews of Modern Physics, 47(3) (July, 1975), pages 649-657
“A tuning range of 1800 A, from the near
ultraviolet to the yellow, has been obtained from an
acidic solution of 4-methyl-umbelliferone....”
C. V. Shank, A. Dienes, A.M. Trozzolo, and J.A. Myer
Applied Physics Letters 16(10) (1970), page 405
A. Dienes, C.V. Shank, and R.L. Kohn
IEEE J. Quant. Electr. vQE-9, 833 (1973)
the Joss Research Institute
Contact Information: