(25 December, 2007, ff)
This page describes my ongoing attempt to make porcelain that is more translucent than bone china. This, no surprise, is a nontrivial endeavor. Before I came to Joss Research, I had already developed a porcelain that is about as good as decent English bone china, and is easier to work with. It looks about like this:
That isn’t bad; in fact, it is slightly more translucent than Southern Ice at the same thickness, and as you can see, it is plastic enough to throw on a wheel. (I have to do things to it to achieve the required degree of plasticity, but we can get into that later.)
I have continued to think about the issues involved in translucency, and have reached some conclusions. A few things appear inescapable; for example, it is quite clear that bubbles and voids are not going to be good for translucency. The refractive index of aluminosilicate glass (the majority constituent of porcelain) is in the vicinity of 1.515, whereas the index of air is close enough to 1.000 as to make no difference to us as potters. Any bubble or void will cause reflections, which bounce light around inside the clay body instead of letting it pass through. The further it travels inside, the more chance that it will be absorbed; moreover, if it doesn’t go straight through, it makes the body appear milky even if it does eventually work its way through and escape on the far side. (Light that goes back out the same side it entered on does not contribute to translucency!)
At least one reference tells me that potash feldspar is better than soda spar for porcelain, because even though potash spar makes larger bubbles, it makes a lot fewer of them than soda spar, and the result is less milky.
Aside from bubbles, what interferes with the passage of light through a fired piece of porcelain?
It is clear that some impurities, particularly iron and titanium, which are very common, are among the culprits. In oxidation, both of them contribute a yellow to brown coloration. In reduction, they are even worse — they combine by charge transfer to form a deep blue color. (This is, in fact, the same color you see in sapphires and in Rutile Blue glazes.) While we tend to see blue-white colors as being whiter than yellow-white colors, they are not necessarily brighter, and likewise a blue-white porcelain is not necessarily more translucent than a yellow-white one. My porcelain actually has a faint greenish color when I fire it in reduction; I have not yet attempted to figure out the cause.
The description of ordinary porcelain involves only three materials: kaolin, feldspar, and “filler”. The most common filler is silica, which in the US is available as powdered quartz. (I understand that flint, a microcrystalline form of silica, is available in England; but even though many US potters use the term, I have never actually seen flint for sale here as a pottery material.)
[I add a small amount of MgO to my mix, as it slightly improves the translucency. This effect is mentioned in the English translation of a book by Hermann Salmang. I took the book out of the library in 1996 or 1997, and I don’t seem to have written down the English title (though I think it had porcelain in it), the original German title, or the publication date, so I am not sure which of his books it was.]
Early in the course of this effort I decided not to add silica to my porcelain body, for reasons that are not important here. The result is a body that is about half clay and half feldspar, and is probably best described by the old term “Parian”. It corresponds to a position that is in the middle of the bottom line of this triaxial diagram, which I have color-coded to make it a bit easier to read:
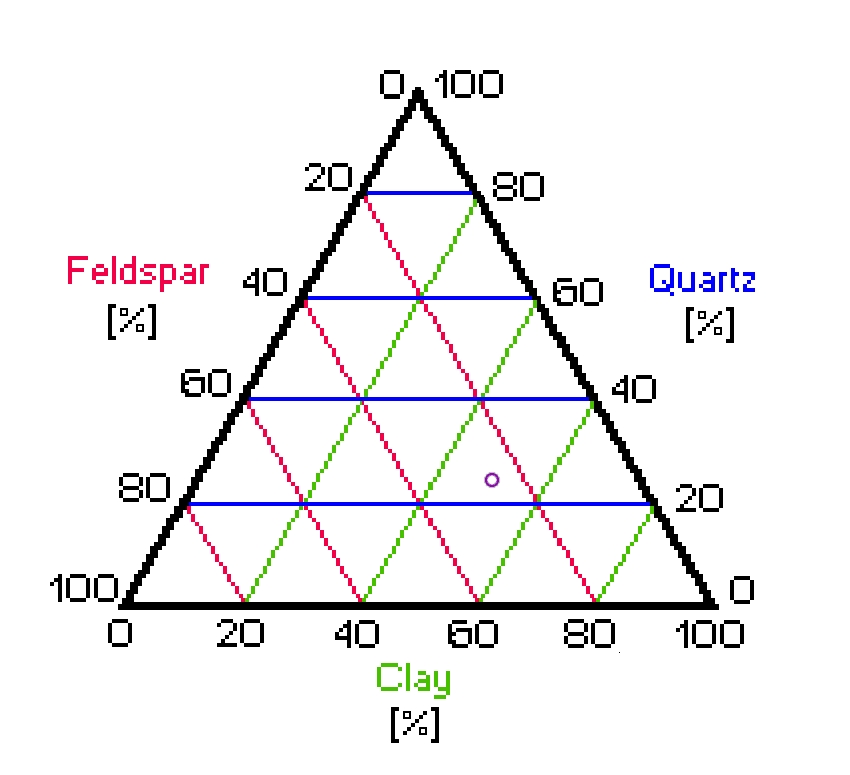
For those who are not familiar with triaxial diagrams:
Quartz sand, which is nearly pure silica, would be very close to the point at the top of the diagram. Pure feldspar would be at the bottom left corner, and pure kaolin would be at the bottom right. To find the composition of a particular mixture, look at the green lines to find the amount of clay, the red lines for the amount of feldspar, and the blue lines for the amount of quartz. (This takes a bit of getting used to, especially with regular published triaxials, which are not color-coded.)
Here are two examples:
Actual formulations (for example, dental porcelains or
electrical porcelains) tend to be somewhat variable, so
they occupy larger regions of the diagram than what is
encompassed by the purple ring, which is just a single
example.
To return to the issue of translucency:
Professor William Carty, of Alfred University, describes formulations like mine as being deficient in silica; they can actually take up more SiO2 than is present in them from the clay and the feldspar. [There is a triaxial diagram for KNaO:Al2O3:SiO2, which makes this more evident.] It turns out that in typical porcelain compositions, some of the added silica goes into solution in the glass that forms, and is not just filler; but much of it fails to go into solution, and Professor Carty suspects that the undissolved material is one of the main hindrances to translucency. I am somewhat doubtful about that, though it is clear that quartz remaining in the body after firing cannot help.
On the other hand, my porcelain does not contain any free quartz, so this is not an issue for me. In fact, at some point I should test small additions of silica to see whether bringing up the amount to the maximum that can fully dissolve will have much effect on the translucency. I would guess that it should help, but I’m not sure by how much.
In any case, porcelain can never be as translucent as glass.
Well over half of a fired porcelain is glass, as I have mentioned above, an aluminosilicate glass that is fairly similar in composition to a clear glaze. Granted, bubbles can be expected to make it milky; but if bubbles were the only problem, I would still expect the fired body to be more translucent than it is.
The remainder of the fired body is mullite, and in thinking about this I decided that the refractive index difference between mullite and aluminosilicate glass was a likely culprit. Although the individual mullite crystals are much smaller than the wavelength of light along their two short axes, they are acicular [needle-shaped], and can easily be more than 1 μ long. In addition they clump together, and the clumps are often considerably larger than the wavelength of light. (Visible light covers a range of wavelengths from ~0.4 to ~0.8 μ, ~400 to ~800 nm. Many people, myself included, can see well beyond 800 nm at the long end; but the sensitivity of the eye to far red wavelengths is quite low, and it seems reasonable to give the spectrum as a single octave.)
Mullite is not isotropic, but both of its refractive
indices are close to 1.645, and it seemed to me that if
I could increase the index of the glassy phase in my
porcelain to that level, the translucency should improve
quite a bit. [This notion was later confirmed by a
diagram I found in Eppler & Eppler, “Glazes
and Glass Coatings”.]
Lanthanum oxide is used in some optical glasses to increase the refractive index, so I decided to try it as an additive to my porcelain. The initial results are not particularly encouraging as far as translucency is concerned, but I am seeing an odd effect in oxidizing firings: perhaps in a way that is similar to the way cerium oxide decolorizes glass, the addition of a small amount of lanthanum oxide seems to make my porcelain less yellow. The effect is subtle, and difficult to see even by eye except in very good light; I have hugely increased the color saturation and contrast in the next photo, in an effort to make it visible here.
On the left are porcelains that do not contain lanthanum; on the right are porcelains with roughly 1% La2O3. The two columns in the middle were fired in oxidation, the two on the edges were fired in reduction. I may have reduced these a bit too much; the tiles that were fired in reduction are considerably darker than I would expect, which I think is a carbon-trapping effect.
Oddly, though it may be difficult to see in this photo, the tiles that were fired in reduction show the opposite effect: the ones that contain lanthanum are actually darker than the ones that do not.
I don’t have an explanation for this yet, but I may be obliged to work out what’s going on here.
In the meanwhile, I am testing other possible
additives, including (at Dr. Carty’s
suggestion) barium carbonate.
(2010.0713)
Neither barium carbonate (which is remarkably stable, and may not have reacted much in my firings) nor barium hydroxide (which played merry hell with the handling characteristics of the clay body, but is quite reactive), seemed to help the translucency.
I have also tried a small amount of gadolinium, but that was not helpful either; as I recall, it caused some bubbling. Cerium, fairly expectably, turned the porcelain yellow. It may be of use as a colorant, but not as an enhancement.
I continue to think about this, and to try other possibilities.
(2010.0713)
Translucency is not, of course, the only issue with porcelain. Modern commercial throwing porcelains, as I have stated elsewhere, are much closer to what the French would have described as “Grès” in earlier eras than they are to fine porcelain; they are not particularly translucent, and the major difference between them and stonewares seems to be the amount of clay in them (stoneware bodies apparently tend to be about 80% clay, whereas porcelains tend to be only 50 or 60% clay) and the fact that they are not typically grogged. The best of them, today, are just about as easy to throw as stoneware, which is quite an achievement.
In order to create a fine porcelain body that is throwable, you have to jump through a few hoops. The first thing I do with mine is to slurry all of the mineral ingredients together. Then I put it in the jar mill for an hour. My understanding is that this accomplishes two or three things: first, it deaggregates the kaolin and the feldspar. Second, it coats the crystals of feldspar with kaolin particles, which prevents them from reaggregating. Third, it dramatically changes the distribution of kaolin particle size, which is one of the keys to plasticity. My kaolin has large average particle size and narrow size range, and it is almost nonplastic. After the slurry has been in the jar mill for an hour or so, I can pour it out on plaster and get a clay body that is almost workable. At that point, the addition of a small amount of a carefully chosen organic polymer is enough to make it quite usable.
The polymer I have been using since early 1997 (I have been working on this project since very shortly after I started doing pottery) is called poly(ethylene oxide) or polyoxyethylene. It is almost the same as polyethylene glycol, but has much higher average molecular weight, and is available in a variety of chain lengths. (You can use eggwhite instead of fancy expensive commercial stuff if you are willing to throw all of your clay within a day or so, before it starts to rot. In fact, eggwhite was the first thing I found that actually worked; most of the other materials I tried were abject failures.)
The advantage of using an organic material as a plasticizer is that it burns out during bisque firing, and cannot interfere with translucency. Bentonite and ball clay, which are used in commercial bodies, tend to introduce iron and titanium, which we are trying to avoid.
One drawback is that if you add too much polymer you get something that behaves about like pizza dough. The trick is to use only just enough to obtain plasticity. It is limitedly possible to tame out the rubberiness by adding other polymers, but that has its own difficulties, and I find it far better to keep the polymer additions to a minimum.
More as it transpires...
My email address is a@b.com, where a is my first name (just jon, only 3 letters, no “h”), and b is joss.
My phone number is +1 240 604 4495.
Last modified: Thu Jun 23 16:02:52 CDT 2016